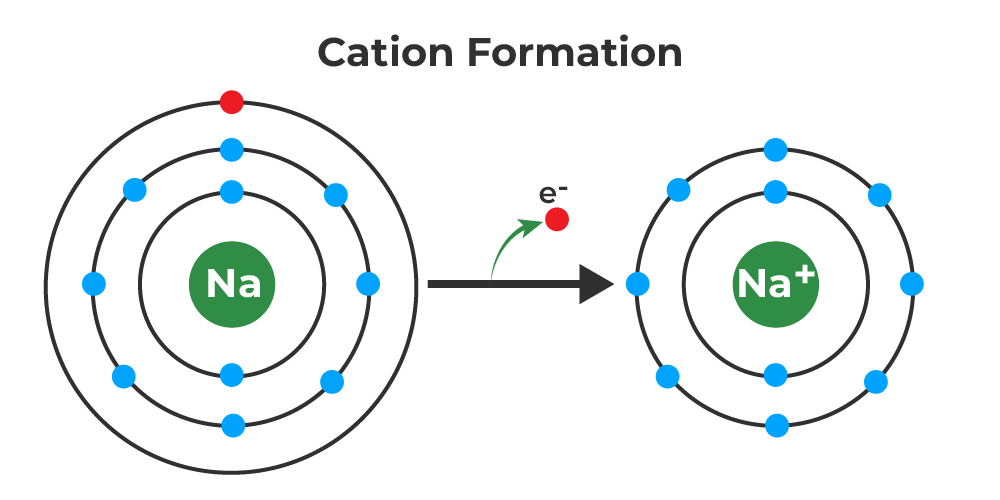
How Are Cations Formed
How Are Cations Formed - The attraction between the positive and negative charges causes the cations and anions to come together and form a stable, bonded structure. Then, the ph was raised to about 8 and h2s was again bubbled through the solution. When an atom shares one or more electrons with another atom d. Cations are formed due to the loss of electrons. Conversely, when an atom gains electrons, it forms an anion which has a negative charge. When an electron is removed, the resulting positive ion has a smaller size compared to the original atom because the number of electrons is now less than the number of protons. Cations are formed when atoms lose electrons, resulting in a net positive charge.
Anions, on the other hand, are formed when atoms gain electrons, resulting in a net negative charge. Cations are formed due to the loss of electrons. When an atom shares one or more electrons with another atom d. Then, the ph was raised to about 8 and h2s was again bubbled through the solution.
Conversely, when an atom gains electrons, it forms an anion which has a negative charge. When an atom shares one or more electrons with another atom d. A cation is a positively charged ion formed when an atom loses one or more electrons. The correct statement among the options is 'cations form when an atom loses electrons'. Cations are formed due to the loss of electrons. When an electron is removed, the resulting positive ion has a smaller size compared to the original atom because the number of electrons is now less than the number of protons.
It's not gain electrons because electrons are negative. When an atom loses one or more electrons In the ionic bond, cation atom is a metal atom and that was formed by the transfer of electron. A precipitate formed and was filtered off. The correct answer is d) smaller than the atom from which they are formed.
The correct statement among the options is 'cations form when an atom loses electrons'. Finally, the solution was treated with a. Conversely, when an atom gains electrons, it forms an anion which has a negative charge. When an atom shares one or more electrons with another atom d.
When An Atom Shares One Or More Electrons With Another Atom D.
In this bond cations are formed in a sea of electrons. A solution containing a mixture of metal cations was treated with dilute hcl and no precipitate formed. Cations are formed due to the loss of electrons. Positive ions, also known as cations, are formed when electrons are removed from atoms.
Cations Are Formed When A Metal Loses Electrons, And A Nonmetal Gains Those Electrons.
The correct answer is d) smaller than the atom from which they are formed. Anions, on the other hand, are formed when atoms gain electrons, resulting in a net negative charge. Next, h2s was bubbled through the acidic solution. When an electron is removed, the resulting positive ion has a smaller size compared to the original atom because the number of electrons is now less than the number of protons.
A Precipitate Again Formed And Was Filtered Off.
When one or more electrons of an atom disintegrate b. It's not gain electrons because electrons are negative. Cations are formed by losing electrons cations are basically positive ions it's not lose or gain protons because atoms can't lose or gain protons. Metallic bonds are the bonds which are having intermediate bond strength and formed by metal atoms only.
In Chemistry, Ions Can Have Either A Positive Charge, Known As Cations, Formed When Atoms Lose Electrons, Or A Negative Charge, Called Anions, Formed When Atoms Gain Electrons.
Finally, the solution was treated with a. Cations are formed when atoms lose electrons, resulting in a net positive charge. When an atom gains one or more electrons from other atoms c. The correct statement among the options is 'cations form when an atom loses electrons'.
When an electron is removed, the resulting positive ion has a smaller size compared to the original atom because the number of electrons is now less than the number of protons. Metallic bonds are the bonds which are having intermediate bond strength and formed by metal atoms only. When an atom shares one or more electrons with another atom d. When one or more electrons of an atom disintegrate b. In chemistry, ions can have either a positive charge, known as cations, formed when atoms lose electrons, or a negative charge, called anions, formed when atoms gain electrons.