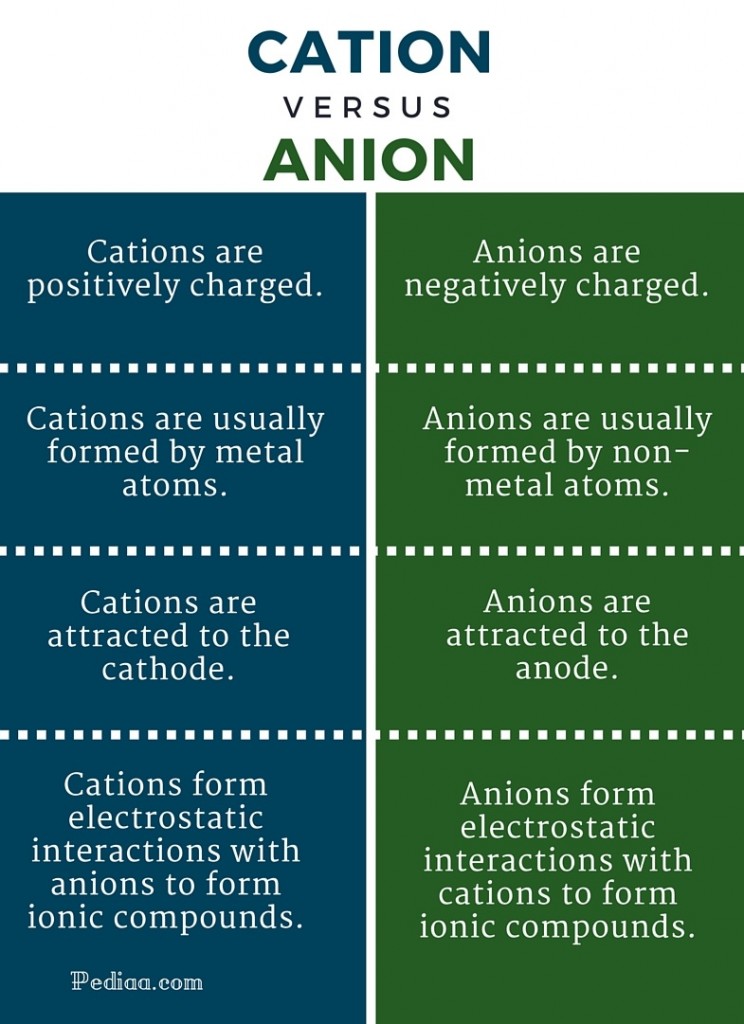
How Is A Cation Formed
How Is A Cation Formed - How many valence electrons does the sodium atom have? The resulting cation has the electron configuration of the noble gas atom in the row above it in the periodic table. Symbolize and name main group cations. The energy required to do this is called the ionization energy. A sodium atom loses one electron to form a sodium cation. Group 1 elements tend to lose 1 electron in reactions, resulting in cations with a change of 1+. H 3 o + ammonium:
Cations are formed when an atom loses one or more electrons. Nh 4 + because an electron is removed to form a cation, the cation of an atom can be smaller than the neutral atom. Groups 1 and 2 elements form cations. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for example) to strip it away from the attraction of the nucleus.
How many valence electrons does the sodium atom have? The energy required to do this is called the ionization energy. Groups 1 and 2 elements form cations. When writing the charge on the ion, remember to put the number before the positive or negative symbol (2+). The word “cation” comes from the greek word ánō, which means “up.” examples of cations include: In many parts of the country, the water contains high concentrations of minerals that stain clothes, build up deposits on bathtubs and water heaters, and create problems with soap foaming properly.
Cation charges are indicated with a superscript following the chemical symbol. It can easily donate to acquire a stable noble gas configuration. Nh 4 + because an electron is removed to form a cation, the cation of an atom can be smaller than the neutral atom. Group 1 elements tend to lose 1 electron in reactions, resulting in cations with a change of 1+. Cations form when an atom loses one or more electrons.
The energy required to do this is called the ionization energy. A sodium atom loses one electron to form a sodium cation. Explain how neutral atoms ionize to form cations. How many valence electrons does the sodium atom have?
Explain How Neutral Atoms Ionize To Form Cations.
How many valence electrons does the sodium atom have? H 3 o + ammonium: When writing the charge on the ion, remember to put the number before the positive or negative symbol (2+). The resulting cation has the electron configuration of the noble gas atom in the row above it in the periodic table.
Cations Are Formed By The Process Of Ionization When Sufficient Energy Is Given To The Electron (By Light Of A High Enough Energy, For Example) To Strip It Away From The Attraction Of The Nucleus.
In many parts of the country, the water contains high concentrations of minerals that stain clothes, build up deposits on bathtubs and water heaters, and create problems with soap foaming properly. Which atom is the sodium ion isoelectronic with? Nh 4 + because an electron is removed to form a cation, the cation of an atom can be smaller than the neutral atom. It can easily donate to acquire a stable noble gas configuration.
Cations Are Named According To The Parent Element.
Cation charges are indicated with a superscript following the chemical symbol. A sodium atom loses one electron to form a sodium cation. Cations are ions that have a positive charge. The resulting cation has the electron configuration of the noble gas atom in the row above it in the periodic table.
Groups 1 And 2 Elements Form Cations.
Cations form when an atom loses one or more electrons. The energy required to do this is called the ionization energy. The charge on a cation is equal to the number of electrons lost. Determine the charges achieved when main group metals ionize.
Determine the charges achieved when main group metals ionize. How many valence electrons does the sodium atom have? Cations form when an atom loses one or more electrons. Group 1 elements tend to lose 1 electron in reactions, resulting in cations with a change of 1+. Cations are formed by the process of ionization when sufficient energy is given to the electron (by light of a high enough energy, for example) to strip it away from the attraction of the nucleus.

:max_bytes(150000):strip_icc()/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)