How Many Covalent Bonds Will Carbon Form
How Many Covalent Bonds Will Carbon Form - These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Carbon forms covalent bonds with atoms of carbon or other elements. Moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. There is a great diversity of carbon compounds, ranging in size from just one to thousands of atoms. This is summarized in the table below. In respiration oxygen rejoins carbohydrates, to form carbon dioxide and water again.
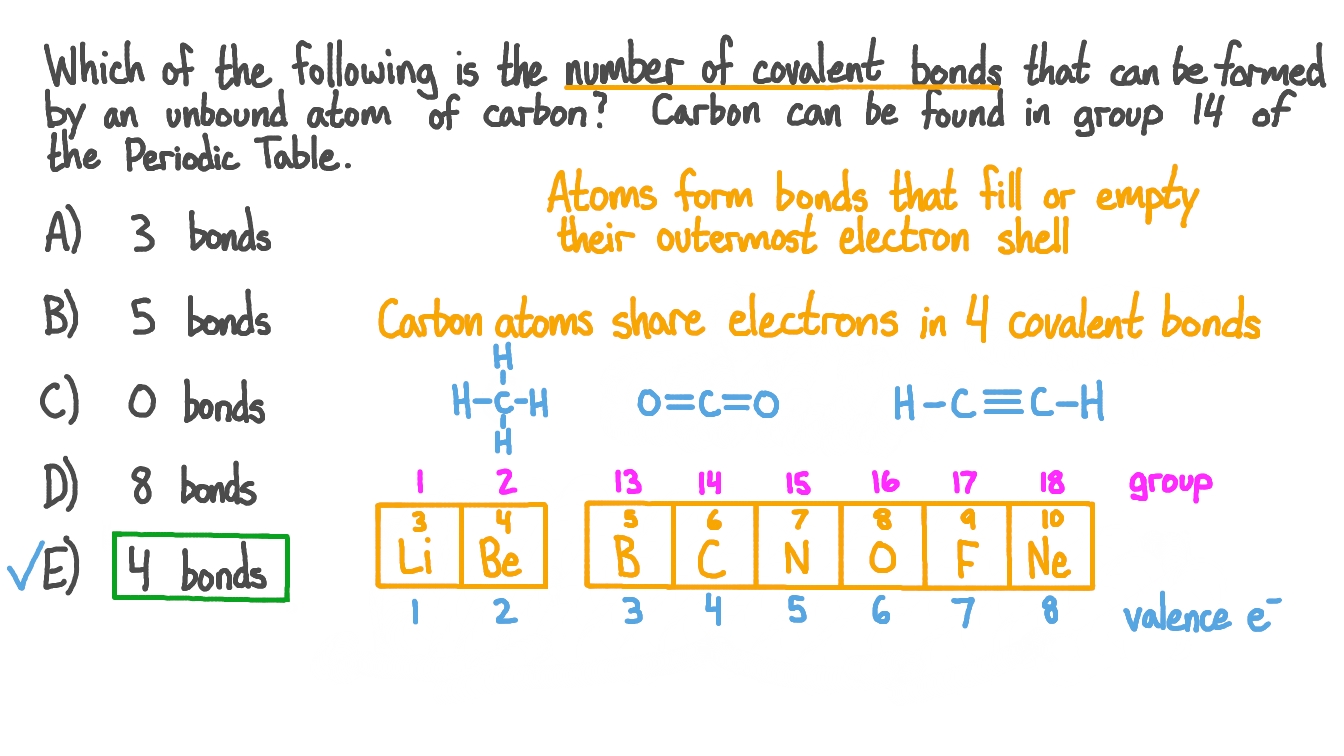
Well, carbon can form up to four covalent bonds. The energy released in this reaction is made available for the cells. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules.
Because hydrogen only needs two. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). The energy released in this reaction is made available for the cells. In respiration oxygen rejoins carbohydrates, to form carbon dioxide and water again. Carbon forms polar covalent bonds with elements that have a. Carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in the figure below.
With hydrogen, nitrogen, oxygen, and other heteroatoms. Each block with a number indicates the number of covalent bonds formed by. This is summarized in the table below. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). In molecules, there is a pattern to the number of covalent bonds that different atoms can form.
Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. A bond composed of two electrons, one from each of the two atoms. Carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in the figure below. The valence electrons are arranged in a balanced pattern providing four bonding sites for.
Carbon Forms Polar Covalent Bonds With Elements That Have A.
Carbons electron configuration shows us 6 total electrons with 4 valence electrons. Carbon can form nonpolar covalent (pure covalent) bonds when it bonds to itself, as in graphene and diamond. In respiration oxygen rejoins carbohydrates, to form carbon dioxide and water again. If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be.
Well, Carbon Can Form Up To Four Covalent Bonds.
For example, diamond, a form of carbon with each carbon atom covalently bonded to four other carbon atoms, is one of the best known thermal conductors. This is summarized in the table below. Carbon, with four valence electrons, forms covalent bonds to four neighboring carbon atoms arranged toward the corners of a tetrahedron, as shown in the figure below. The valence electrons are arranged in a balanced pattern providing four bonding sites for.
The Number Of Electrons Required To Obtain An Octet Determines The Number Of Covalent Bonds An Atom Can Form.
These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. Carbon forms covalent bonds with atoms of carbon or other elements. Each block with a number indicates the number of covalent bonds formed by.
How Can Carbon Form 4 Bonds?
In molecules, there is a pattern to the number of covalent bonds that different atoms can form. A bond composed of two electrons, one from each of the two atoms. With hydrogen, nitrogen, oxygen, and other heteroatoms. The energy released in this reaction is made available for the cells.
The number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. A bond composed of two electrons, one from each of the two atoms. The energy released in this reaction is made available for the cells. In molecules, there is a pattern to the number of covalent bonds that different atoms can form. There is a great diversity of carbon compounds, ranging in size from just one to thousands of atoms.





![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/large/693c5ecd-56f0-42a1-aba9-2e5a5be31300/covalent-bonding-in-co2---teachoo.jpg)
![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/large/6f2704db-da18-4198-8dd5-4dc81ae3ac22/carbon-sharing-electrons-with-hydrogen---teachoo.jpg)