What Type Of Electron Is Available To Form Bonds
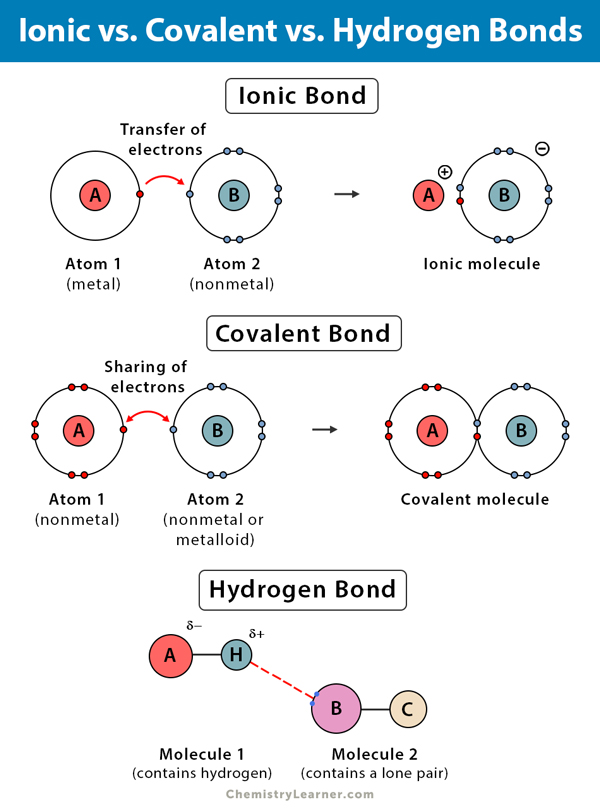
What Type Of Electron Is Available To Form Bonds - Covalent bonds are either polar covalent bonds or nonpolar covalent bonds. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only. When electrons are shared between two atoms, they form a covalent bond. What type of electron is available to form bonds?. Main group valence electrons are located in the s and p orbitals and are responsible for the formation of. Valence electrons are the outermost electrons in the electron orbits. The bond may result from the electrostatic force between oppositely charged ions.
Main group valence electrons are located in the s and p orbitals and are responsible for the formation of. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons. Hence, a bond can form by the pairing of each hydrogen electron with an oxygen electron and the overlap of the orbitals they occupy. The electrons that are available to form bonds are known as valence electrons.
When electrons are shared between two atoms, they form a covalent bond. Valence electrons are the outermost electrons in the electron orbits. The valence electron of the metal (the electropositive species) transfers to the valence shell of the nonmetal (the electronegative species), forming a chemical bond. Covalent bonds are either polar covalent bonds or nonpolar covalent bonds. When atoms come together to form chemical bonds, they do so by sharing, gaining, or losing valence electrons. The interaction of valence electrons between atoms allows for the formation.
Covalent bonds are either polar covalent bonds or nonpolar covalent bonds. Main group valence electrons are located in the s and p orbitals and are responsible for the formation of. A nonpolar covalent bond forms when two atoms with the same electronegativity share electrons. The electrons that are available to form bonds are known as valence electrons. Chemical bonding tends to be of two types;
The valence electron of the metal (the electropositive species) transfers to the valence shell of the nonmetal (the electronegative species), forming a chemical bond. Covalent, in which electrons are shared between atoms, and ionic in which two. When electrons are shared between two atoms, they form a covalent bond. The bond may result from the electrostatic force between oppositely charged ions.
Covalent Bonds Are Either Polar Covalent Bonds Or Nonpolar Covalent Bonds.
Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons. In conclusion, the type of electron available to form bonds is the valence electron. Chemical bonding tends to be of two types; When atoms come together to form chemical bonds, they do so by sharing, gaining, or losing valence electrons.
Hence, A Bond Can Form By The Pairing Of Each Hydrogen Electron With An Oxygen Electron And The Overlap Of The Orbitals They Occupy.
Learn about covalent and ionic bonding, how they arise from the need for electrons or the need to remove electrons, and how they store and release energy. See questions and answers on. Main group valence electrons are located in the s and p orbitals and are responsible for the formation of. The electron distribution arising from each.
The Electrons That Are Available To Form Bonds Are Known As Valence Electrons.
Covalent, in which electrons are shared between atoms, and ionic in which two. The bond may result from the electrostatic force between oppositely charged ions. A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. When electrons are shared between two atoms, they form a covalent bond.
Valence Electrons Are The Outermost Electrons In The Electron Orbits.
The valence electron of the metal (the electropositive species) transfers to the valence shell of the nonmetal (the electronegative species), forming a chemical bond. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only. What type of electron is available to form bonds?.
When atoms come together to form chemical bonds, they do so by sharing, gaining, or losing valence electrons. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons. When electrons are shared between two atoms, they make a bond called a covalent bond. The electrons that are available to form bonds are known as valence electrons. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only.